Thinking as Simulation of Behaviour: an Associationist
View of Cognitive Function
Germund Hesslow
Department of Physiological Sciences
University of Lund
Address:
BMC F10
Tornavägen 10
SE-221 84 LUND
Sweden
e-mail: Germund.Hesslow@mphy.lu.se
Introduction
It may be said that cognitive science rests on the assumption that human behaviour cannot be understood by taking only perceptual and motor processes into account and that distinct cognitive mechanisms are required to explain behaviour. Yet, developments in several fields during the last couple of decades suggest that cognitive and motor brain mechanisms are intimately connected, perhaps indistinguishable. Among these are emerging ideas about embodied cognition (Clark, 1997; Varela et al., 1991), findings of a close connection between imaging and perception and the evidence for involvement of brain motor structures in cognition.
This somewhat paradoxical situation can be resolved by a combination of some old ideas about covert behaviour and sensory reactivation, originally formulated by British empiricist philosophers in the 18th century (Hume, 1888) and their associationist descendants (Bain, 1868). These ideas were abandoned for a long time, but have later been revived and are now supported by extensive experimental evidence.
The simulation hypothesis, as we shall call it, states that thinking consists of simulated interaction with the environment (Hesslow, 1994) and rests on the following three core assumptions:
1) Simulation of actions. We can activate pre-motor areas in the frontal lobes in a way that resembles activity during a normal action but does not cause any overt movement.
2) Simulation of perception. Imagining that one perceives something is essentially the same as actually perceiving it, but the perceptual activity is generated by the brain itself rather than by external stimuli.
3) Anticipation. There are associative mechanisms that enable
both behavioural and perceptual activity to elicit other perceptual activity
in the sensory areas of the brain. Most importantly, a simulated
action can elicit perceptual activity that resembles the activity that
would have occurred if the action had actually been performed.
Simulation of behaviour
In his remarkably insightful book The Senses and the Intellect
from 1855, Alexander Bain suggested that thinking is essentially the same
sort of thing as behaviour, but of a covert or ‘weak’ form that does not
activate the body and is therefore invisible to an external observer (Bain,
1868). Speech, said Bain, is "nothing else than a sort of whisper... instead
of the full-spoken utterance." (p.347). This idea, which was accepted by
Watson and other behaviourists (Skinner, 1953; Watson, 1930), was thought
to have been disproved when it was shown that subjects paralysed by curare
were still able to think. (Smith et al., 1947) It may have been
prematurely rejected, however and a slightly modified version of it has
lived on.

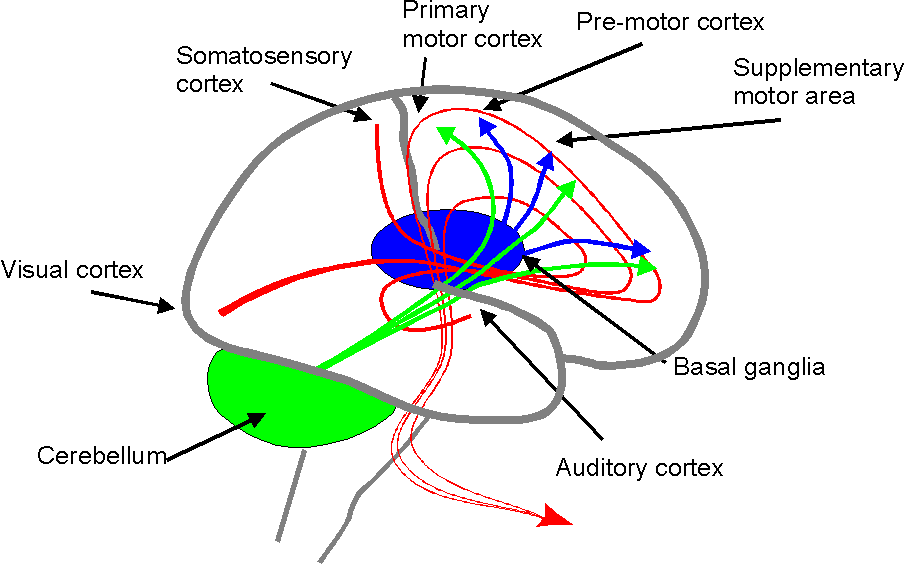
Figure 1. The main signal flow through the brain. Behaviour is generated in a hierarchical fashion in the frontal lobes. Activity in sensory cortex is signalled to the rostral parts of the frontal lobe. The main signal flow then goes caudally through supplementary and premotor cortex to the primary motor cortex. Single muscles and elementary movements, say a finger flexion or a speech sound, are controlled by neurones in the primary motor cortex. More complex movements, which require temporally organised activation of several muscles, say gripping an object or saying a word, are elicited by higher-level command signals generated by more rostral neurones. More complex actions, such as making coffee or uttering a sentence, are generated even more rostrally and in the prefrontal cortex only the most global aspects of behaviour are controlled. The frontal lobes are constantly interacting with the cerebellum and the basal ganglia. The former receives input from many sensory and motor cortical areas and also peripheral input and sends fast correction signals to all levels of the frontal lobes. The basal ganglia also project to all levels of the behaviour generating mechanisms in the frontal lobes.
Behaviour is generated in a hierarchical fashion in the frontal lobes (Fig. 1) with ‘global’ action commands arising in the frontal areas and specific elementary movements in the primary motor cortex. The idea that behaviour can be simulated, means that the frontal lobe activity, which prepares and initiates a normal action may occur while the primary motor cortex output is suppressed. There is now an impressive body of evidence that supports this hypothesis (for reviews, see Jeannerod (1994) and Jeannerod & Frak (1999). Notice, however, that the simulation hypothesis does not only say that covert and actual movements utilise a common neural substrate – it says that the two processes are essentially the same.
Behavioural experiments have demonstrated a number of striking parallels between simulated and actual movements. Many studies show that the time it takes to simulate a simple motor task, such as reading the alphabet, for instance, corresponds to the time it takes to actually perform the same task (Landauer, 1962). Decety et al. had subjects walk blindfolded to familiar places, while indicating with a stop watch when they started and when they thought that they had reached the goal. When they later imagined walking to the same places, there was a very good correspondence between the time a particular subject used for the imagined and the actual walk (Decety et al., 1989).
The most impressive evidence for the similarity between ‘mental’ and physical movement has been obtained by using modern imaging techniques. A number of such studies show that subjects instructed to simulate a certain movement will activate roughly the same brain areas that are active when these movements are performed physically. Ingvar and Philipsson (Ingvar & Philipsson, 1977)showed that when subjects were instructed to simulate and physically perform hand movements, both types of activity correlated with increased neural activity in the rostral parts of the frontal lobes, while only the overt movements activated the primary motor cortex. A large number of later studies have reported similar observations (Roland et al., 1980b; Roland et al., 1980a). Although there may be some subtle differences between imagined and executed movements, later studies using PET and fMRI have confirmed activation of premotor and supplementary motor areas during imagined movements (Deiber et al., 1998; Lotze et al., 1999; Rao et al., 1993).
It has been shown fairly recently that the excitability of neurones
in the primary motor cortex is also increased during simulation of movement.
If transcranial magnetic stimulation is applied to a restricted area in
the motor cortex so that a certain muscle group is activated, before versus
after a subject has been instructed to imagine the same movement, the stimulation
will elicit a stronger reaction in the second case (Fadiga et al.,
1999).
Simulation of perception
The idea that we can simulate perception by activating the sensory areas of the brain in a way that resembles the activity normally initiated by the sense organs, was hinted at by Hume ( 1888) and explicitly formulated by several writers during the 19th century (Bain, 1868; James, 1890), but the evidence for it is fairly recent (For more extensive reviews e.g. Farah, (1988) and (Kosslyn, 1994)).
Many investigators have compared the effects of concrete manipulations
of physical objects with the corresponding ‘mental’ manipulations. In the
Shepard & Metzler ‘mental rotation task’ (Fig 2), for instance, subjects
were assumed to determine whether pairs of three-dimensional objects were
identical by rotating one of them until it could be viewed from the same
perspective as the reference figure. The time it took to find the solution
proved to be closely correlated with the degree of rotation, rather as
if the subjects were looking at rotating objects and had to wait for the
degree of rotation at which they could be compared (Shepard & Metzler,
1971).
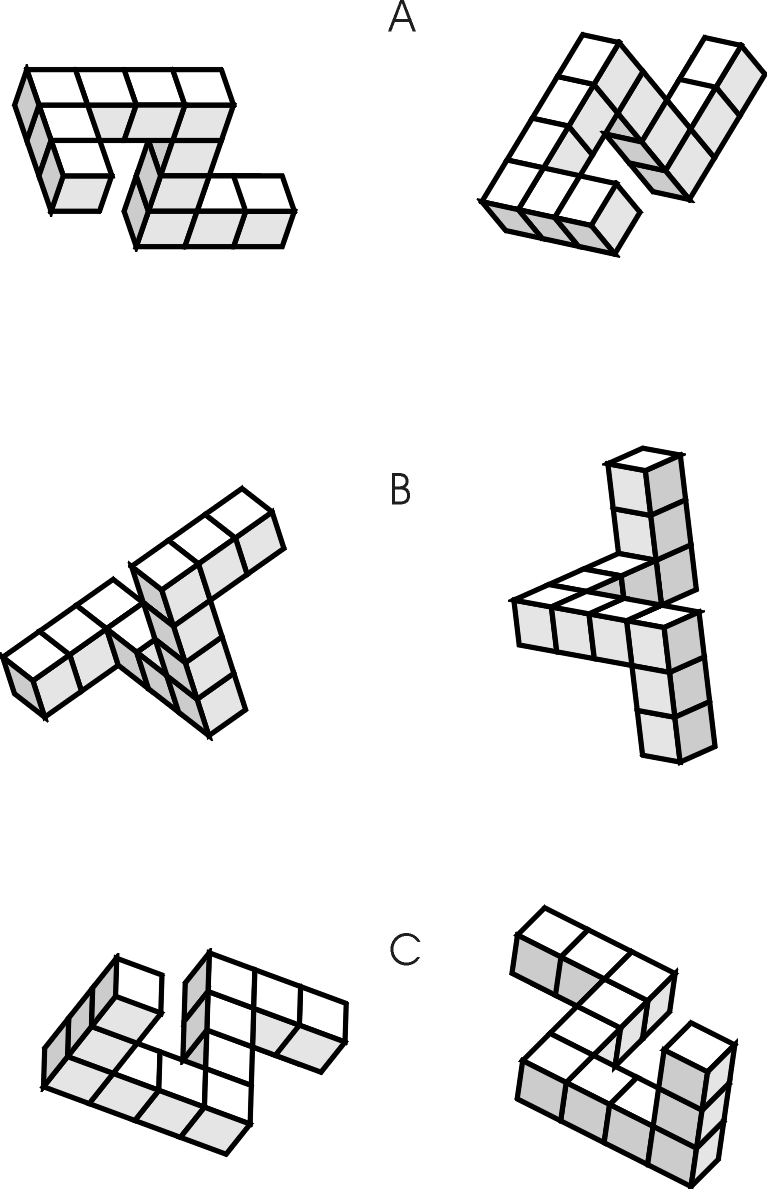
Figure 2. Shepard & Metzler’s ‘Mental rotation task’. Subjects were shown pairs of drawings of three-dimensional objects and asked whether the members of a pair were identical. The task can be solved for physical objects by rotating one of them until they can be viewed from the same perspective, but in this case the subjects had to perform the rotation "mentally".
This and other similar experiments have been interpreted as evidence that imagery utilises the same mechanisms as the visual system. Some investigators have gone further and claimed that ‘images’ are real (independently existing entities or brain states) and have ‘depictive’ or ‘pictorial’ properties. This is a controversial issue (Kosslyn, 1994; Pylyshyn, 1984), that need not concern us here. The simulation hypothesis makes no assumptions about the nature of either imagery or perception except that the perceptual mechanisms can be activated internally.
Another source of evidence has been patients who are blind because of lesions of various parts of the visual cortex. Although the symptoms of such damage are quite variable, many patients with cortical blindness, have also lost their ability to form visual images (Farah, 1988). Another striking example is a report on patients with unilateral damage to the parietal cortex, that causes an inability to notice and respond to stimuli in the contralateral visual field (‘neglect syndrome’). Such patients were asked to imagine that they were standing on one side of a familiar square in their home city and describe what they could remember. They could only describe buildings on the right side of the square, relative to the imagined vantage point. When they were asked to imagine that they were standing at the opposite side of the square, they could describe the buildings which were now in the right visual field, but which they had not been able to describe the first time (Bisiach & Luzzatti, 1978).
The most compelling support for the idea, that imaging utilises the same mechanisms as perception, has been obtained while measuring activity in various parts of the brain when subjects imagine a stimulus. In one of the earliest of these experiments, electrical activity was measured in both visual and somatosensory cortex while subjects imagined various visual and tactile stimuli. When asked to imagine light flashes, activity increased specifically in the visual cortex, while imagining that someone was touching their arm, increased activity in the somatosensory cortex (Davidson & Schwartz, 1977).
Similar results come from a rapidly growing number of modern imaging studies. Imagining a visual stimulus or performing a task that requires visualisation, is accompanied by increased activity the primary visual cortex (Le Bihan et al., 1993). Actually the size of the imagined stimulus determined the exact locus of activation (Kosslyn et al., 1993; Tootell et al., 1998).
The same relation seems to hold for specialised secondary visual areas. A region of the occipito-temporal cortex called the fusiform face area is activated both when we see faces (Kanwisher et al., 1997) and also when we imagine them (O'Craven & Kanwisher, 2000). Lesions which include this area impair face recognition (Damasio et al., 1990) and also the ability to imagine faces (Young et al., 1994).
Anticipation
There is evidence both from animal behaviour and human imaging studies, that perceptual simulation can be elicited by other perceptual activity (Gallistel, 1990; Nyberg et al., 2000), but here we will consider the possibility that perceptual simulation can be elicited from the frontal lobes by preparations for actions.
What we perceive is to a large extent determined by our
own behaviour. Visual input is changed when we move our heads or eyes;
tactile stimulation is generated in the hands by manipulating objects.
The sensory consequences of behaviour are to a large extent predictable.
The simulation hypothesis postulates the existence of an associative mechanism
that enables the preparatory stages of an action to elicit sensory activity
that resembles the activity that would normally be caused by the completed
overt behaviour (Fig. 2). A plausible neural substrate for such a mechanism
is the extensive fibre projection from the anterior lobe to all parts of
sensory cortex, although a partial alternative route would be via the cerebellum.
Very little is known about the function of these pathways, but there is
physiological evidence from monkeys that neurones in polysensory cortex
can be modulated by movement (Hietanen & Perrett, 1996).
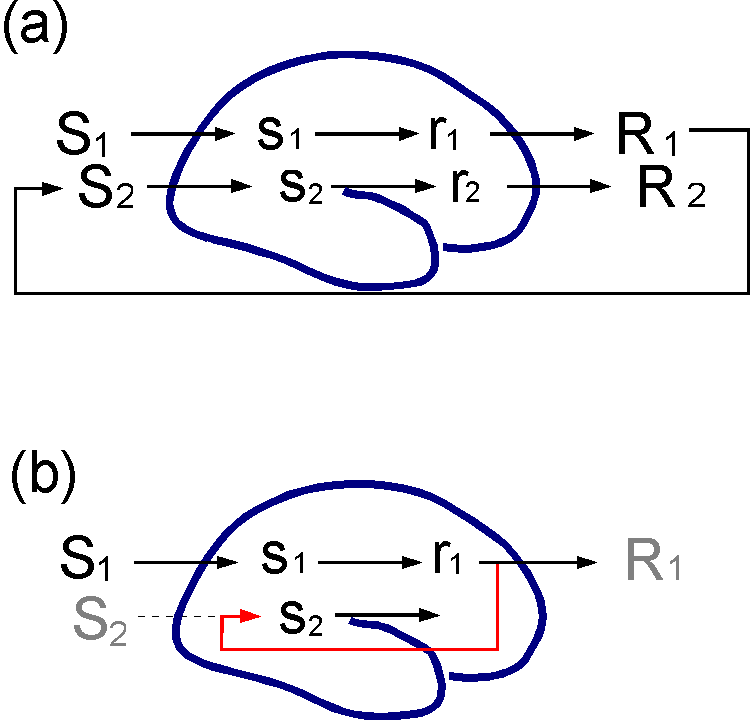
Figure 3. Anticipation. (a) A situation S1 elicits activity s1 in sensory cortex that leads to preparation for action r1 and then to an action or response R1. The response changes the situation in which the organism finds itself into S2, which then causes new perceptual activity etc. The relations between behaviour and consequent stimuli are often predictable. (b) The simulation hypothesis assumes that associations (in red) can be formed such that r1 will generate s2, the most probable sensory consequence of R1, before or even in the absence of R1.
Anticipation does not necessarily involve the sensory cortex. In classical conditioning of motor responses, the association is made between the conditioned stimulus and output neurones in the cerebellum (Hesslow G. & Yeo, 2002). However, an anticipation mechanism of the sort suggested above has many obvious advantages. In particular it would enable the organism to interrupt activity that threatens to have dangerous consequences in situations where classical conditioning in itself would be insufficient.
A good example is the seemingly goal-directed behaviour exemplified by various ‘revaluation’ experiments. Take for instance the behaviour of rats in one of Tolman’s classical T-maze experiments. In a classic experiment, Tolman and Gleitman (1949) let a rat freely explore a T-maze with a dark goal box in the left arm of the maze and a light goal box to the right. Both boxes contained food. The rat was then placed in dark chamber, similar to the left goal box, and subjected to electrical foot shocks. When the rat was later placed in the T-maze, it went directly to the right goal box and did not enter the left arm of the maze, in spite of the fact that it had never been punished for this behaviour and that left and right turns had been equally reinforced. It looked as if it had access to a map of the maze and inferred that an unpleasant experience awaited it in the left goal box.
Why do the rats perform responses that have never been
differentially reinforced? A simple explanation is, that when the rat reaches
the choice point in the T-maze, it will actually sometimes initiate
the alternative ‘bad’ response, that is, walking towards the aversive stimulus.
When this behaviour is still at the preparatory stage, however, it elicits
the usual sensory consequences, learned during the initial exploration
of the T-maze, namely the sight of the dark goal box. This would elicit
conditioned anxiety, which suppresses completion of the initiated behaviour.
There is thus a fairly unproblematic and concrete sense in which the rat
‘anticipates’ the consequences of walking to the left. The selectionist
account of the ‘Law of Effect’ could still be right, but it is simulated
responses rather than executed ones that are selected.
Simulating chains of behaviour
Once the mechanism of anticipation is in place, there
is nothing to prevent the appearance of long chains of simulated responses
and perceptions. A simulated action in the frontal lobe can generate a
simulated perception of its probable consequences in the sensory cortex.
This activity may serve as a stimulus for a new response and so on (Fig.
3). By such simulated interaction with the external world, an organism
could evalute not only single responses but whole courses of action before
putting them to physical and more dangerous tests.
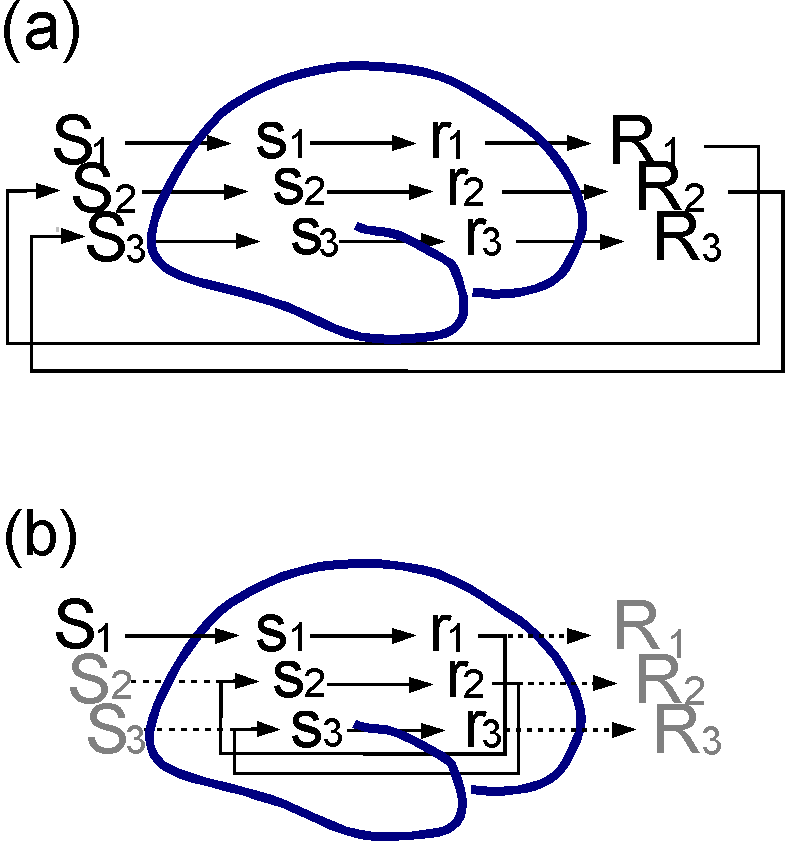
Figure 4. Simulation of behavioural chain. (a)
Behavioural chain, where each response, R1, R2, R3...
generates a new situation, S2, S3...,
that elicits a new response etc. (b) An anticipation mechanism
will enable an organism to simulate the behavioural chain by performing
covert responses and the perceptual activity elicited by the anticipation
mechanism. Even if no overt movements and no sensory consequences occur,
a large part of what goes on inside the organism will resemble the events
arising during actual interaction with the environment.
It is tempting at this point to assume that there must be some part of the brain or some autonomous agent or ‘self’ that ‘performs’ the simulation by ‘using’ frontal lobes, but that is not what is being suggested here. The anticipation mechanism will ensure that most actions are accompanied by probable perceptual consequences, so that during normal behaviour, we will always, ‘in our thoughts’ be a few steps ahead of the actual events. Nor do we need to posit an independent agent that ‘evaluates’ the simulation. The (simulated) sensory events will elicit previously learned emotional consequences, which can guide future behaviour either by reinforcing or punishing simulated actions, which may transfer to overt actions, or by serving as discriminative stimuli.
This kind of simulation is a plausible interpretation
of the problem-solving process in tasks like the Tower of London (Fig.
5). The subject may test a certain move by simulating moving one ball,
thereby generating a perception of the new configuration that can function
as a stimulus for the next move, and so on until a good or bad result is
eventually achieved. Consistent with this suggestion, subjects working
with the Tower of London task activate premotor areas, including the supplementary
motor area, and sensory areas (in particular the parietal and occipital
cortices) (Baker et al., 1996; Dagher et al., 1999).
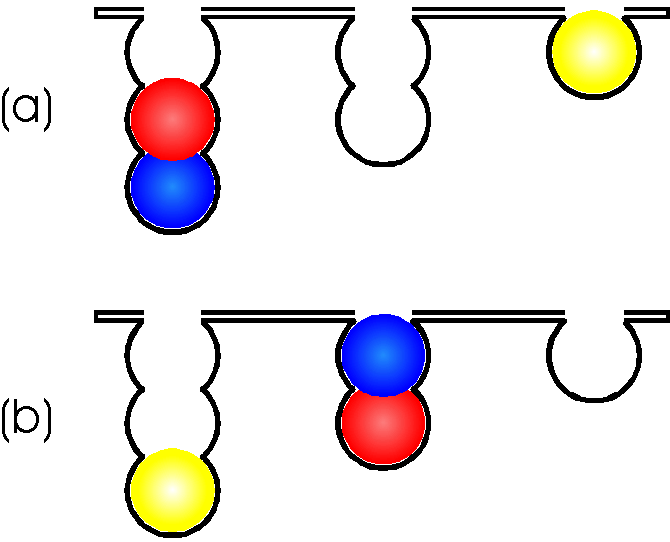
Figure 5. The Tower of London (Shallice, 1988).There are three pouches which can hold one, two and three balls respectively. The task is to move the differently coloured balls, one at a time in a minimum number of moves, between the pouches in the lower panel so that the resulting pattern matches that of the upper panel. A ball can only be moved if there is no other ball above it and it cannot be moved higher in a pouch if there is no ball below on which it can rest. The task can be done physically, with actual balls, or "mentally". For instance, the subject may be shown the figure here and asked to indicate the minimum number of moves necessary to solve the problem.
A similar interpretation can be given of mental rotation. Subjects trying to solve the Shepard & Metzler task, may simulate rotating the figures, thereby activating motor areas of the frontal lobes and, as a consequence, sensory cortex. This has indeed been found. EEG recordings have revealed activation of premotor and parietal cortical areas (Williams & Rippon, 1995). fMRI studies have similarly found activation of the supplementary motor area as well as of the parietal cortex during mental rotation (Cohen et al., 1996; Richter et al., 2000).
The essential process could in principle be applied to
many other types of cognitive processes. If the preparation of a verbal
response can generate activity in the auditory cortex, it should be possible
to ‘hear’ it before it results in overt speech, whether or not it ever
does. Thus, a conversation between two individuals, or between an individual
and himself could be simulated internally (Hesslow, 1994).
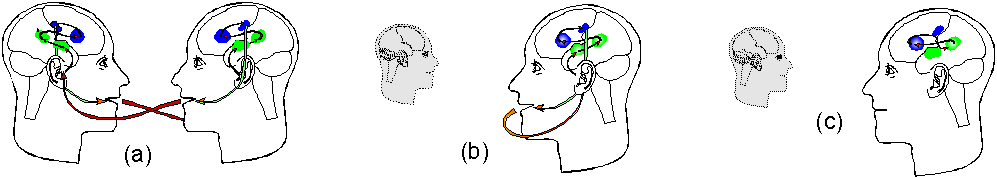
Figure 6. Internalisation of conversation. (a)
In a conversation, we can perform a verbal response, such as responding
to a question, without being conscious until quite a bit later. (b) We
can also talk to ourselves. (c) If the verbal response can also be fed
into some part of the auditory cortex or Wernicke’s area, we can also speak
to ourselves internally. Thus, a process that consists of unconscious components,
can give rise to an inner conversation and what we might call ‘verbal thinking’.
Further advantages of the simulation hypothesis
In addition to the empirical evidence described above, I would like to point out four attractive features of the simulation hypothesis.
Role of the cerebellum and basal ganglia in cognitive function
The simulation hypothesis also makes sense of the accumulating data showing that ‘motor’ structures such as the cerebellum and the basal ganglia appear to be involved in cognitive tasks. The cerebellum is activated during imagined movements (Decety et al., 1990; Ryding et al., 1993), in the Tower of London task (Baker et al., 1996) and during mental rotation (Parsons et al., 1995; Parsons & Fox, 1997). Cerebellar lesions seem to cause various forms of cognitive impairments (Dominey et al., 1995) (Schmahmann, 1997).
The basal ganglia, particularly the striatum, are also
activated during various cognitive tasks such as the Tower of London (Baker
et
al., 1996; Dagher et al., 1999) and performance in this task
is impaired or altered in patients with Parkinson’s disease (Dagher
et
al., 2001; Morris et al., 1988).
No extravagant ontological assumptions
The simulation hypothesis requires no far-reaching assumptions
about the existence of ‘images’, ‘representations’, ‘mental models’ or
any other of the abstract entities so often postulated by current cognitive
science. Simulation is conceptually firmly tied to basic behavioural and
neural processes. That simple associations between actions and their sensory
consequences is sufficient for internal simulation, is demonstrated by
a recent robot simulation. Jirenhed and Ziemke have shown that a robot
can successfully navigate in a simple environment using only predicted
stimuli as input (Jirenhed et al., 2001; Ziemke et al., 2002).
No evolutionary leaps
Although there are some obvious quantitative differences,
the general construction of the human brain is quite similar to that of
other, cognitively simpler, mammals, such as rats and cows, and it has
not evolved any radically novel circuits for dealing with higher cognitive
functions. From an evolutionary point of view, therefore, cognitive functions
are likely to be based on other more basic functions of the brain. It is
a strength of the simulation hypothesis, that it can account for cognitive
functions in terms of neural mechanisms that are present in rats and cows
and that have evolved because they enable organisms to move about, find
food and reproduce.
The inner world and consciousness
Perhaps the most exciting aspect of internal simulation is that it suggests a mechanism for generating the inner world that we associate with consciousness. There are many problems of consciousness, but surely one of them concerns the existence of an inner world of experience that does not immediately depend on external input. How does this inner world arise? The simulation hypothesis provides a simple and straightforward answer. Since simulation of behaviour and perception will be accompanied by internally generated sensory input resembling perceptions of the external world, it will inevitably be accompanied by the experience of an inner, non-physical, world.
Conclusion
The specific evidence for the first two assumptions of the simulation hypothesis, simulation of action and simulation of perception, is quite strong. The third assumption is less well supported by specific evidence but it is highly plausible and there is no evidence against it. The most compelling argument in favour of the simulation hypothesis in general is the fact that it explains and makes sense of wide range of phenomena and that it does so without relying on extravagant assumptions about underlying brain mechanisms.
Acknowledgements: I am grateful to several people
for valuable discussions on the topic of this paper, among them Rodney
Cotterill, Håkan Eriksson, Dan-Anders Jirenhed and Tom Ziemke. The
work was supported by The Swedish Medical Research Council (09899).
Bain, A. (1868). The Senses and the Intellect, 3 ed. Longmans, Green & Co, London.
Baker, S. C., Rogers, A. D., Owen, A. M., Frith, C. D., Dolan, R. J., Frackowiak, R. S., & Robbins, T. W. (1996). Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia 34, 515-526.
Bisiach, E. & Luzzatti, C. (1978). Unilateral neglect of representational space. Cortex 14, 129-133.
Clark, A. (1997). Being There: Putting Brain, Body, and World Together Again MIT Press, Cambridge, Mass.
Cohen, M. S., Kosslyn, S. M., Breiter, H. C., DiGirolamo, W. L., Thompson, W. L., Anderson, A. K., Bookheimer, S. Y., Rosen, B. R., & Belliveau, J. W. (1996). Changes in cortical activity during mental rotation. A mapping study using functional MRI. Brain 119, 89-100.
Dagher, A., Owen, A. M., Boecker, H., & Brooks, D. J. (1999). Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain 122, 1973-1987.
Dagher, A., Owen, A. M., Boecker, H., & Brooks, D. J. (2001). The role of the striatum and hippocampus in planning: a PET activation study in Parkinson's disease. Brain 124, 1020-1032.
Damasio, A. R., Tranel, D., & Damasio, H. (1990). Face agnosia and the neural substrates of memory. Annual Review of Neuroscience 13, 89-109.
Davidson, R. J. & Schwartz, G. E. (1977). Brain mechanisms subserving self-generated imagery: Electrophysiological specificity and patterning. Psychophysiology 14, 598-601.
Decety, J., Jeannerod, M., & Prablanc, C. (1989). The timing of mentally represented actions. Behavioural Brain Research 34, 35-42.
Decety, J., Sjöholm, H., Ryding, E., Stenberg, G., & Ingvar, D. H. (1990). The cerebellum participates in cognitive activity: Tomographic measurements of regional cerebral blood flow. Brain Research 535, 313-317.
Deiber, M. P., Ibanez, V., Honda, M. S. N., Raman, R., & Hallett, M. (1998). Cerebral processes related to visuomotor imagery and generation of simple finger movement studied with positron emission tomography. Neuroimage 7, 73-85.
Dominey, P. F., Decety, J., Broussolle, E., Chazot, G., & Jeannerod, M. (1995). Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson patients. Neuropsychologia 33, 727-741.
Fadiga, L., Buccino, G., Craighero, L., Fogassi, L., Gallese, V., & Pavesi, G. (1999). Corticospinal excitability is specifically modulated by motor imagery. A magnetic stimulation study. Neuropsychologia 37, 147-158.
Farah, M. J. (1988). Is visual imagery really visual? Overlooked evidence from neuropsychology. Psychological Review 95, 307-317.
Gallistel, C. R. (1990). The Organisation of Learning MIT Press, Cambridge.
Hesslow G. & Yeo, C. H. (2002). The Functional Anatomy of Skeletal Conditioning. In A Neuroscientist's Guide to Classical Conditioning, ed. Moore, J. W., Springer, New York.
Hesslow, G. (1994). Will neuroscience explain consciousness? Journal of Theoretical Biology 171, 29-39.
Hietanen, J. K. & Perrett, D. I. (1996). Motion sensitive cells in the macuaqe superior temporal polysensory area: response discrimination between self-generated and externally pattern motion. Behavioral Brain Research 76, 155-167.
Hume, D. (1888). A Treatise of Human Nature Oxford University Press, Oxford.
Ingvar, D. H. & Philipsson, L. (1977). Distribution of the cerebral blood flow in the dominant hemisphere during motor ideation and motor performance. Annals of Neurology 2 , 230-237.
James, W. (1890). Principles of Psychology Macmillan (republished by Dover 1950).
Jeannerod, M. (1994). The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences Vol 17, 187-245.
Jeannerod, M. & Frak, V. (1999). Mental imaging of motor activity in humans. Current Opinion in Neurobiology 9, 735-739.
Jirenhed, D.-A., Hesslow, G., & Ziemke, T. Exploring internal simulation of perception in a mobile robot. Arras, Baerveldt Balkenius Burgard & Siegwart. Lund University Cognitive Studies 86, 107-113. 2001. Lund. Fourth European Workshop on Advanced Mobile Robots (Eurobot '01) - Proceedings.
Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience 17, 4302-4311.
Kosslyn, S. M. (1994). Image and Brain: The Resolution of the Imagery Debate MIT Press, Cambridge.
Kosslyn, S. M., Alpert, N. M., Thompson, W. L., Maljkovic, V., Weise, S. W., Chabris, C. F., Hamilton , S. E., Rauch, S. L., & Buonanno, F. S. (1993). Visual Mental Imagery Activates Topographically Organized Visual Cortex: PET Investigations. Journal of Cognitive Neuroscience 5, 263-287.
Landauer, T. K. (1962). Rate of implicit speech. Perceptual and Motor Skills 15, 646.
Le Bihan, D., Turner, R., Zeffiro, T. A., Cuénod, C. A., Jezzard, P., & Bonnerod, V. (1993). Activation of human primary visual cortex during visual recall: A megnetic resonance imaging study. Proceedings of the National Academy of Sciences 90, 11802-11805.
Lotze, M., Montoya, P., Erb, M., Hülsmann, E., Flor, H., Klose, U., Birbaumer, N., & Grodd, W. (1999). Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. Journal of Cognitive Neuroscience 11, 491-501.
Morris, R. G., Downes, J. J., Sahakian, B. J., Evenden, J. L., Heald, A., & Robbins, T. W. (1988). Planning and spatial working memory in Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 51, 757-766.
Nyberg, L., Habib, R., McIntosh, & Tulving, E. (2000). Reactivation of encoding-related brain activity during memory retrieval. Proceedings of the National Academy of Sciences 97 , 11120-11124.
O'Craven, K. M. & Kanwisher, N. (2000). Mental Imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience 12, 1013-1023.
Parsons, L. M. & Fox, P. T. (1997). Sensory and cognitive functions. In The Cerebellum and Cognition, ed. Schmahmann, J. D., pp. 255-271. Academic Press, New York.
Parsons, L. M., Fox, P. T., Downs, J. H., Glass, T., Hirsch, T. B., Martin, C. C., Jerabek, P. A., & Lancaster, J. L. (1995). Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375, 54-58.
Pylyshyn, Z. W. (1984). Computation and Cognition MIT Press, Cambridge, MA.
Rao, S. M., Binder, J. R., Bandettini, P. A., Hammeke, T. A., Yetkin, F. Z., Jesmanowicz, A., Lisk, L. M., Morris, G. L., Meuller, W. M., Estikowski, L. D., Wong, E. C., Haughton, V. M., & Hyde, J. S. (1993). Functional magnetic resonance imaging of complex human movements. Neurology 43, 2311-2318.
Richter, W., Somorjai, R., Summers, R., Jarmasz, M., Menon, R. S., Gati, J. S., Georgopoulos, A. P., Tegeler, C., Ugurbil, K., & Kim, S. G. (2000). Motor area activity during mental rotation studied by time-resolved fMRI. Journal of Cognitive Neuroscience 12, 310-320.
Roland, P. E., Lassen, N. A., Larsen, B., & Skinhoj, E. (1980a). Supplementary motor area and other cortical areas in organisation of voluntary movements in man. Journal of Neurophysiology 43, 118-136.
Roland, P. E., Skinhoj, E., Lassen, N. A., & Larsen, B. (1980b). Different cortical areas in man in organisation of voluntary movements in extrapersonal space. Journal of Neurophysiology 43, 137-150.
Ryding, E., Decety, J., Sjöholm, H., Stenberg, G., & Ingvar, D. H. (1993). Motor imagery activates the cerebellum regionally: A SPECT rCBFstudy with 99m Tc-HMPAO. Cognitive Brain Research 1, 94-99.
Schmahmann, J. D. e. al. (1997). The Cerebellum and Cognition. Academic Press, New York.
Shallice, T. (1988). Specific impairments of planning. Philosophical Transactions of the Royal Society of London B 298, 199-209.
Shepard, R. N. & Metzler, J. (1971). Mental rotation of three-dimensional objects. Science 171, 701-703.
Skinner, B. F. (1953). Science and human behavior Macmillan, New York.
Smith, S. M., Brown, H. O., Toman, J. E., & Goodman, L. S. (1947). The lack of cerebral effects of d-tubercurarine. Anesthesiology8, 1-14.
Tolman,E.C. and Gleitman,H. (1949). Studies in learning and motivation: I. Equal reinforcements in both end-boxes, followed by shock in one end-box. Journal of Experimental Psychology 39, 810-819.
Tootell, R. B. H., Hadjikhani, N. K., Mendola, J. D., Marrett, S., & Dale, A. M. (1998). From retinotopy to recognition: fMRI in human visual cortex. Trends in Neuroscience 2, 174-183.
Varela, F. J., Thompson, E., & Rosch, E. (1991). The Embodied Mind: Cognitive Science and Human Experience MIT Press, Cambridge.
Watson, J. B. (1930). Behaviorism, 2nd ed. Norton (Originally published 1924), New York.
Williams, J. D. & Rippon, G. (1995). Psychophysiological correlates of dynamic imagery. British Journal of Psychology 86, 283-300.
Young, A. W., Humphreys, G. W., Riddoch, M. J., Hellawell, D. J., & DeHaan, E. H. (1994). Recognition impairments and face imagery. Neuropsychologia 32, 693-702.
Ziemke, T., Jirenhed, D.-A., & Hesslow G. (2002). Blind Adaptive Behavior based on Internal Simulation of Perception. Technical report HS-IDA-TR-02-001.